Controlling electronic properties of MoS2/graphene oxide heterojunctions for enhancing photocatalytic performance: the role of oxygen†
Abstract
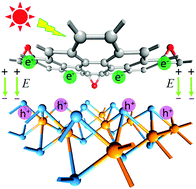
The manipulation of the constituents of novel hetero-photocatalysts is an effective method for improving photocatalytic efficiency, but a theoretical understanding of the relationship between interlayer interaction and photocatalytic activity is still lacking. Herein, the interfacial interactions and electronic properties of MoS2/graphene oxide (GO) heterojunctions with various O concentrations were explored systematically by first-principles calculations. The results indicate that MoS2 and GO can form a stable van der Waals heterojunction, and enhance the built-in internal electric field from GO to the MoS2 surface with the increase in O concentration after interfacial equilibrium. It is inferred that the photogenerated electrons and holes naturally accumulate in the conduction band of GO and the valence band of MoS2, respectively, under the built-in internal electric field driving, indicating the formation of direct Z-scheme heterojunctions. In addition, a red shift of the light absorption edge and the shift up of the conduction band edge of MoS2/GO heterojunctions are observed with an increase in O concentration. It can be concluded that the O atom plays a crucial role in the energy band alignment of MoS2/GO heterojunctions for the improvement of photocatalytic performance. These results are beneficial to understand and design layered MoS2/GO photocatalytic systems or as cocatalysts with other semiconductors.


 Please wait while we load your content...
Please wait while we load your content...