From E to Z and back again: reversible photoisomerisation of an isolated charge-tagged azobenzene
Abstract
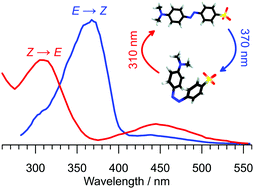
Substituted azobenzenes serve as chromophores and actuators in a wide range of molecular photoswitches. Here, tandem ion mobility spectrometry coupled with laser excitation is used to investigate the photoisomerisation of selected E and Z isomers of the charge-tagged azobenzene, methyl orange. Both isomers display a weak S1(nπ*) photoisomerisation response in the blue part of the spectrum peaking at 440 nm and a more intense S2(ππ*) photoisomerisation response in the near-UV with maxima at 370 and 310 nm for the E and Z isomers, respectively. The 60 nm separation between the S2(ππ*) photo-response maxima for the two isomers allows them to be separately addressed in the gas phase and to be reversibly photoisomerised using different colours of light. This is an essential characteristic of an ideal photoswitch. The study demonstrates that a sequence of light pulses at different stages in an ion mobility spectrometer can be deployed to generate and probe isomers that cannot be electrosprayed directly from solution or produced through collisions in the ion source.


 Please wait while we load your content...
Please wait while we load your content...