Mechanistic insights into the formation of oxenium ions and radical intermediates through the photolysis of phenylhydroxylamine and its derivatives†
Abstract
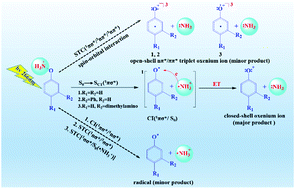
The photolysis of photoprecursors to produce oxenium ions has been the subject of extensive experimental studies from femtosecond to microsecond time scales. However, mechanistic insights into the generation of activated intermediate species remain elusive. Herein, we present a theoretical investigation to comprehensively elucidate the possible reaction channels for the formation of oxenium ions and radical intermediates at the multi-configuration perturbation level of theory. Computational results show that photo-initiated electron donation from the phenyl moiety to the repulsive N–O σ* orbital leads to the formation of a diradical intermediate in ground state, and further triggers intramolecular electron transfer from the phenyl moiety to the ammonia radical cation (˙NH3+). This affords closed-shell singlet oxenium ions and neutral :NH3 as the major products. However, the generation of open-shell triplet outcomes is shown to rely on the energetically accessible single–triplet crossings and spin–orbital interaction among the involved electronic states. Taken together, these data can be used to determine the electronic structures and related properties, as well as reactivities, of oxenium ions and radicals generated by the photolysis of phenylhydroxylamine and its derivatives.


 Please wait while we load your content...
Please wait while we load your content...