Estimating the mean first passage time of protein misfolding†
Abstract
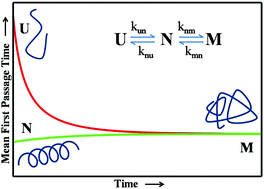
Most theoretical and experimental studies confirm that proteins fold in the time scale of microseconds to milliseconds, but the kinetics of the protein misfolding remains largely unexplored. The kinetics of unfolding–folding–misfolding equilibrium in proteins is formulated in the analytical framework of the Master equation. The folded, unfolded and the misfolded state are characterized in terms of their respective contacts. The Mean First Passage Time (MFPT) to acquire the misfolded conformation from the native or folded state is derived from this equation with different boundary conditions. The MFPT is found to be practically independent of the length of the protein, the number of native contacts and the rate constant for the misfolded to the folded state. The results obtained from the survival probability are directly correlated to the age of onset and appearance of misfolding diseases in humans.


 Please wait while we load your content...
Please wait while we load your content...