Predicting anisotropic thermal displacements for hydrogens from solid-state NMR: a study on hydrogen bonding in polymorphs of palmitic acid†
Abstract
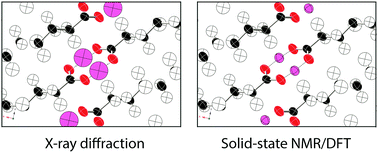
The hydrogen-bonding environments at the COOH moiety in eight polycrystalline polymorphs of palmitic acid are explored using solid-state NMR. Although most phases have no previously reported crystal structure, measured 13C chemical shift tensors for COOH moieties, combined with DFT modeling establish that all phases crystallize with a cyclic dimer (R22(8)) hydrogen bonding arrangement. Phases A2, Bm and Em have localized OH hydrogens while phase C has a dynamically disordered OH hydrogen. The phase designated As is a mix of five forms, including 27.4% of Bm and four novel phases not fully characterized here due to insufficient sample mass. For phases A2, Bm, Em, and C the anisotropic uncertainties in the COOH hydrogen atom positions are established using a Monte Carlo sampling scheme. Sampled points are retained or rejected at the ±1σ level based upon agreement of DFT computed 13COOH tensors with experimental values. The collection of retained hydrogen positions bear a remarkable resemblance to the anisotropic displacement parameters (i.e. thermal ellipsoids) from diffraction studies. We posit that this similarity is no mere coincidence and that the two are fundamentally related. The volumes of NMR-derived anisotropic displacement ellipsoids for phases with localized OH hydrogens are 4.1 times smaller than those derived from single crystal X-ray diffraction and 1.8 times smaller than the volume of benchmark single crystal neutron diffraction values.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...