Comparative photophysical investigation of doubly-emissive photochromic-fluorescent diarylethenes†
Abstract
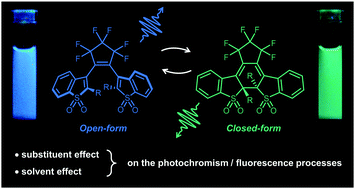
Diarylethene molecules showing photochromism and fluorescence properties in both open and closed forms, associated with two different emission colors, are very promising for applications involving ratiometric emissive photoswitches. We report here a complete study on the competition between the multiple photophysical processes involved in the excited states for two sulfone derivatives of benzothiophene-based diarylethene molecules, only differing by the substituent groups on their reactive carbon (methyl for DAE-Me and ethyl for DAE-Et). Steady-state and time-resolved spectroscopy, combined with DFT and TD-DFT calculations, allow a complete determination of the kinetic constants leading to fluorescence and photoreaction pathways in different solvents, and enlighten the specific role of the substituent group in the photophysical properties due to a shielding effect against the solvation environment. The predominant role of the non-radiative deactivation processes in such a family of molecules is shown, and a tentative excited state mechanistic scheme is proposed based on femtosecond transient absorption experiments performed on the closed forms.


 Please wait while we load your content...
Please wait while we load your content...