2-Chloromalonaldehyde, a model system of resonance-assisted hydrogen bonding: vibrational investigation†
Abstract
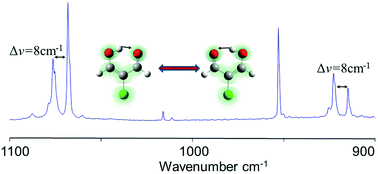
The chelated enol isomer of 2-chloromalonaldehyde (2-ClMA) is experimentally characterized for the first time by IR and Raman spectroscopies. The spectra are obtained by trapping the molecule in cryogenic matrices and analyzed with the assistance of theoretical calculations. Experiments were performed in argon, neon and para-hydrogen matrices. The results highlight puzzling matrix effects, beyond site effects, which are interpreted as due to a tunneling splitting of the vibrational levels related to the proton transfer along the internal hydrogen bond (IHB). 2-ClMA is thus one of the very few molecules in which the H tunneling has been observed in cryogenic matrices. The comparison with its parent molecule (malonaldehyde) shows experimentally and theoretically the weakening of the IHB upon chlorination, with a reduced cooperative effect in the resonance assisted hydrogen bond. In addition, the Cl substitution induces an important stabilization of two open enol conformers. These two open forms appear in the spectra of as-deposited samples, meaning that, in contrast with other well-studied molecules of the same family (β-dialdehydes and β-diketones), they are present in the gas phase at room temperature.


 Please wait while we load your content...
Please wait while we load your content...