Propane and propane–water interactions: a study at cryogenic temperatures†
Abstract
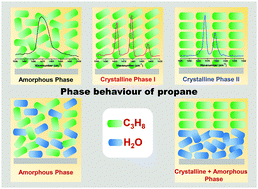
The phase transition of solid propane and a propane–water mixture under ultrahigh vacuum has been investigated using reflection absorption infrared spectroscopy (RAIRS) and temperature-programmed desorption mass spectrometry (TPD-MS). Here, the investigation is divided into two sections: the phase transition of pure propane and the interaction of propane with water. RAIR spectra of pure propane reveal an unknown crystalline phase at 50 K (phase I), which gradually converts to a known crystalline phase (phase II) at higher temperature. This conversion is associated with certain kinetics. Co-deposition of water and propane restricts the amorphous to crystalline phase transition, while sequential deposition (H2O@C3H8; propane over predeposited water) does not hinder it. For an alternative sequential deposition (C3H8@H2O; water over predeposited propane), the phase transition is hindered due to diffusional mixing within the given experimental time, which is attributed to the reason behind the restricted phase transition.


 Please wait while we load your content...
Please wait while we load your content...