The aggregation of an alkyl–C60 derivative as a function of concentration, temperature and solvent type†
Abstract
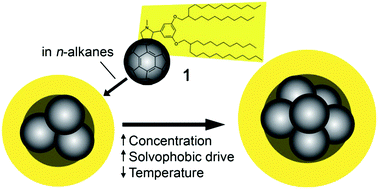
Contrast-variation small-angle neutron scattering (CV-SANS), small-angle X-ray scattering (SAXS), nuclear magnetic resonance (NMR) measurements of diffusion and isothermal titration calorimetry (ITC) are used to gain insight into the aggregation of an alkyl–C60 derivative, molecule 1, in n-hexane, n-decane and toluene as a function of concentration and temperature. Results point to an associative mechanism of aggregation similar to other commonly associating molecules, including non-ionic surfactants or asphaltenes in non-aqueous solvents. Little aggregation is detected in toluene, but small micelle-like structures form in n-alkane solvents, which have a C60-rich core and alkyl-rich shell. The greatest aggregation extent is found in n-hexane, and at 0.1 M the micelles of 1 comprise around 6 molecules at 25 °C. These micelles become smaller when the concentration is lowered, or if the solvent is changed to n-decane. The solution structure is also affected by temperature, with a slightly larger aggregation extent at 10 °C than at 25 °C. At higher concentrations, for example in solutions of 1 above 0.3 M in n-decane, a bicontinuous network becomes apparent. Overall, these findings aid our understanding of the factors driving the assembly of alkyl–π-conjugated hydrophobic amphiphiles such as 1 in solution and thereby represent a step towards the ultimate goal of exploiting this phenomenon to form materials with well-defined order.
- This article is part of the themed collection: Complex molecular systems: supramolecules, biomolecules and interfaces


 Please wait while we load your content...
Please wait while we load your content...