σ-Holes and σ-lumps direct the Lewis basic and acidic interactions of noble metal nanoparticles: introducing regium bonds†
Abstract
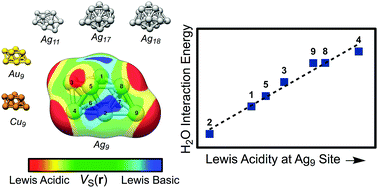
Using local DFT-based probes for electrostatic as well as charge transfer/polarization interactions, we are able to characterize Lewis basic and acidic sites on copper, silver and gold nanoparticles. The predictions obtained using the DFT-probes are compared to the interaction energies of the electron donating (CO, H2O, NH3 and H2S) and the electron accepting (BH3, BF3, HCl [H-down] and Na+) compounds. The probes include the local electron attachment energy [E(r)], the average local ionization energy [Ī(r)], and the electrostatic potential [V(r)] and are evaluated on isodensity surfaces located at distances corresponding to typical interaction distances. These probes have previously been successful in characterizing molecular interactions. Good correlations are found between Lewis acidity and maxima in V(r), appearing as a consequence of σ-holes, as well as minima in E(r), of the noble metal nanoparticles. Similarly are Lewis basic sites successfully described by surface minima in V(r) and Ī(r); the former are indicative of σ-lumps, i.e. regions of enhanced σ-density. The investigated probes are anticipated to function as reliable tools in nanoparticle reactivity and interaction characterization, and may act as suitable descriptors in large-scale screenings for materials of specific properties, e.g. in heterogeneous catalysis. Because of the similarity between the noble metal nanoparticle's interactions with Lewis bases and the concepts of halogen and hydrogen bonding, a new class of bonds is introduced – regium bonds – taking place between a σ-hole of a Cu, Ag or Au compound and an electron donor.


 Please wait while we load your content...
Please wait while we load your content...