On the kinetic decomposition voltage of ternary oxides
Abstract
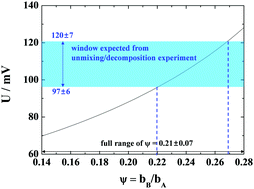
In a previous article the authors reported the kinetic unmixing and decomposition of the ternary oxide NiTiO3 by an externally applied voltage. It was found [J. Chun, et al., J. Appl. Phys., 117, 2015, 124504] that while kinetic unmixing occurred for all magnitudes of the applied voltage, kinetic decomposition occurred only above a certain threshold voltage Ukind. The experimentally determined value of Ukind, however, did not coincide with the thermodynamic expectation according to the definition by Gibbs (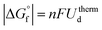 ). In this work the kinetic decomposition voltage Ukind of a ternary oxide ABOν is theoretically derived and compared with the experimental results. It turns out that Ukind depends on the mobility ratio of the cations and agrees with observed results for the system of NiTiO3 within the error bounds.
). In this work the kinetic decomposition voltage Ukind of a ternary oxide ABOν is theoretically derived and compared with the experimental results. It turns out that Ukind depends on the mobility ratio of the cations and agrees with observed results for the system of NiTiO3 within the error bounds.


 Please wait while we load your content...
Please wait while we load your content...