Solution and gas phase evidence of anion binding through the secondary bonding interactions of a bidentate bis-antimony(iii) anion receptor†
Abstract
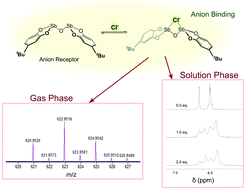
The solution and gas phase halide binding to a bis-antimony(III) anion receptor was studied. This new class of anion receptors utilizes the strong Sb-centered secondary bonding interactions (SBIs) that are formed opposite to the polar Sb–O primary bond. 1H NMR titration data were fitted statistically to binding models and solution-phase binding energetics were extracted, while the formation of anion-to-receptor complexes was observed using ESI-MS. Density functional theory calculations suggest that their affinity towards binding halide anions is mitigated by the strong explicit solvation effect in DMSO, which gives insights into future designs that circumvent direct solvent binding and are anticipated to yield tighter and perhaps more selectivity in anion binding.


 Please wait while we load your content...
Please wait while we load your content...