A theoretical study on the size-dependence of ground-state proton transfer in phenol–ammonia clusters†
Abstract
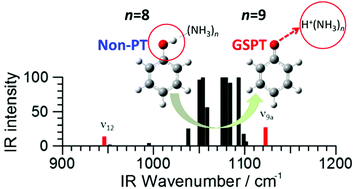
Geometries and infrared (IR) spectra in the mid-IR region of phenol–(ammonia)n (PhOH–(NH3)n) (n = 0–10) clusters have been studied using density functional theory (DFT) to investigate the critical number of solvent molecules necessary to promote ground-state proton transfer (GSPT). For n ≤ 8 clusters, the most stable isomer is a non-proton-transferred (non-PT) structure, and all isomers found within 1.5 kcal mol−1 from it are also non-PT structures. For n = 9, the most stable isomer is also a non-PT structure; however, the second stable isomer is a PT structure, whose relative energy is within the experimental criterion of population (0.7 kcal mol−1). For n = 10, the PT structure is the most stable one. We can therefore estimate that the critical size of GSPT is n = 9. This is confirmed by the fact that these calculated IR spectra are in good accordance with our previous experimental results of mid-IR spectra. It is demonstrated that characteristic changes of the ν9a and ν12 bands in the skeletal vibrational region provide clear information that the GSPT reaction has occurred. It was also found that the shortest distance between the π-ring and the solvent moiety is a good indicator of the PT reaction.
- This article is part of the themed collection: Complex molecular systems: supramolecules, biomolecules and interfaces


 Please wait while we load your content...
Please wait while we load your content...