The C-terminal cytidine deaminase domain of APOBEC3G itself undergoes intersegmental transfer for a target search, as revealed by real-time NMR monitoring†
Abstract
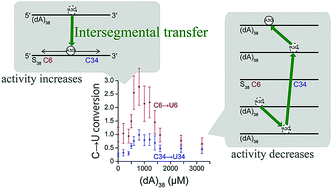
APOBEC3G (A3G), an anti-human immunodeficiency virus 1 factor, deaminates cytidines. We examined deamination of two cytidines located separately on substrate ssDNA by the C-terminal domain (CTD) of A3G using real-time NMR monitoring. The deamination preference between the two cytidines was lost when either the substrate or non-substrate ssDNA concentration increased. When the non-substrate ssDNA concentration increased, the deamination activity first increased, but then decreased. This indicates that even a single domain, A3G-CTD, undergoes intersegmental transfer for a target search.
- This article is part of the themed collections: 2018 PCCP HOT Articles and Complex molecular systems: supramolecules, biomolecules and interfaces


 Please wait while we load your content...
Please wait while we load your content...