Effect of a single water molecule on the HO2 + ClO reaction†
Abstract
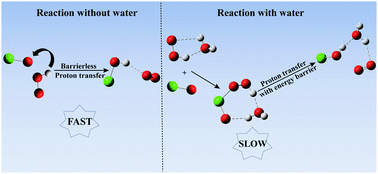
The catalytic effect of a single water molecule on the HO2 + ClO reaction has been investigated at the CCSD(T)/aug-cc-pVTZ//B3LYP-D3/aug-cc-pVDZ level of theory. Four H-abstraction paths and two kinds of products, among which the paths for HOCl + O2 formation are dominant, have been found for the HO2 + ClO reaction without water. The rate constant of the most favorable path for the reaction without water is computed to be 4.53 × 10−12 cm3 molecule−1 s−1 at room temperature, in good agreement with the experiment. In the presence of a water molecule, although the reaction becomes more complex, the dominant products do not change. Four main channels, starting from HO2⋯H2O + ClO, H2O⋯HO2 + ClO, ClO⋯H2O + HO2 and H2O⋯ClO + HO2, are investigated. The most favorable paths, reactions between H2O⋯HO2 and ClO, and between ClO⋯H2O and HO2, are 7–10 and 6–9 orders of magnitude slower than the reaction in the absence of water, respectively. It is concluded that the presence of a single water molecule does not play an important role in enhancing the HO2 + ClO reaction under tropospheric conditions.
- This article is part of the themed collection: Bunsentagung 2018: Kinetics in the Real World


 Please wait while we load your content...
Please wait while we load your content...