Increase in the reduction potential of uranyl upon interaction with graphene oxide surfaces†
Abstract
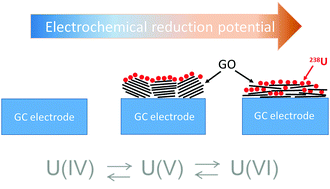
Coordination of uranyl (U(VI)) with carboxylate groups on functionalized graphene oxide (GO) surfaces has been shown to alter the reduction potential of the sorbed uranium ion. A quantitative measure of the reduction potential and qualitative estimation of sorption/desorption processes were conducted using cyclic voltammetry, and the proposed coordination environment was determined using the surface sensitive attenuated total reflection mode of infrared spectroscopy (ATR-FTIR). GO is a nanostructured material possessing a large amount of oxygen-containing functional groups both on basal planes and at the edges, which can form strong surface complexes with radionuclides. The presence of these functional groups on the surface of GO allows efficient immobilization of uranium due to sorption of uranyl (UO22+) to carboxylate, hydroxide, or sulfonate functional groups and the potential for enhanced reduction of U(VI) to more strongly sorbing and insoluble U(IV). Herein, binding of U(VI) to carboxylate groups on the GO surface is proposed as the primary sorption mechanism based on the FTIR study. Furthermore, the coordination of uranium with the surface increases the reduction potential of the U(VI)/U(IV) redox couple as compared to the case of the aqueous U(VI)/U(IV) species. This is consistent with the alteration of the electronic structure of the sorbed ion, which can be determined in our case due to the use of a GO-coated working electrode. Thus, GO-coated glassy carbon electrodes and other semi-conducting electrodes with high ion sorption capacities may provide a means of examining the oxidation/reduction potentials of sorbed ions.


 Please wait while we load your content...
Please wait while we load your content...