Solvate and protic ionic liquids from aza-crown ethers: synthesis, thermal properties, and LCST behavior†
Abstract
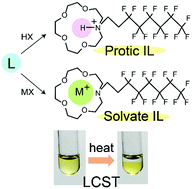
In recent years, solvate and protic ionic liquids (ILs) have attracted much attention. We synthesized both types of ILs from alkyl aza-crown ethers (L = N-propyl-1-aza-15-crown-5 (L1) and N-C6F13C2H4-1-aza-15-crown-5 (L2)). The solvate ILs [ML][Tf2N] (M = Na+, K+) were solids (Tm = 58–68 °C), whereas the solvate ILs [ML][Tf2N] (M = Li+, Ag+) and protic ILs [HL][Tf2N] were liquids with low glass transition temperatures. The ILs containing Na ions were more crystalline and exhibited higher melting points than the other ILs. The decomposition temperatures of the protic ILs were higher than those of the solvate ILs. A protic IL with a paramagnetic anion, [HL1][FeCl4] (Tm = 70.5 °C), was also synthesized and its crystal structure was determined. The solvate ILs [NaL2][X] (X = Cl−, CF3CO2−, TsO−, PhSO3−) exhibited a lower critical solution temperature (LCST)-type behavior in water. The effects of salt addition on the LCST of L2 were also investigated. The LCST of these ILs generally increased with increasing hydrophilicity or basicity of the counter anion. This tendency, which is nearly opposite to that of ILs with quaternary onium cations, is ascribed to the amphiphilic nature of the cation. The corresponding protic ILs did not exhibit LCST behavior.
- This article is part of the themed collection: Complex molecular systems: supramolecules, biomolecules and interfaces


 Please wait while we load your content...
Please wait while we load your content...