Chiral and kryptoracemic Dy(iii) complexes with field-induced single molecule magnet behavior†
Abstract
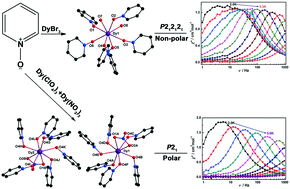
Two mononuclear Dy(III) compounds formulated as [Dy(PNO)6(H2O)2]Br3 (1) and [Dy(PNO)6(NO3)](ClO4)2 (2) have been synthesized and characterized. Single crystal X-ray diffraction studies show that chiral and kryptoracemic structures are formed when different anions are introduced into the system. 1 crystallizes in the non-polar orthorhombic space group P212121, whereas 2 assembles as a kryptoracemate in the polar monoclinic space group P21. The optical activity of the single crystals of these two compounds was confirmed by circular dichroism (CD) studies. Thermal gravimetric analyses (TGA) revealed that they are stable up to 376 K (1) and 428 K (2). Differential scanning calorimetry (DSC) measurements demonstrate the absence of structural phase transitions over the investigated temperature range. Magnetic studies show that both compounds are field-induced single molecule magnets (SMMs) with energy barriers of 36 (±0.8) K for 1 and 32 (±1.4) K for 2. Furthermore, the dielectric and ferroelectric properties of compound 2 were also investigated.


 Please wait while we load your content...
Please wait while we load your content...