Combining complexing agent and solvothermal reaction for the morphology controlled synthesis of (Y,Eu)PO4 crystals with size-dependent photoluminescence†
Abstract
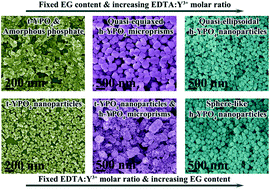
Hexagonally structured YPO4 (h-YPO4) micro/nanocrystals with the multiform morphologies of quasi-equiaxed microprisms, quasi-ellipsoidal nanoparticles, and sphere-like nanoparticles, have been successfully synthesized via a facile solvothermal reaction in the co-presence of appropriate amounts of ethylene glycol (EG) and ethylene diamine tetraacetic acid (EDTA) or citric acid (CA). A series of precisely controlled experiments indicated that EG can significantly amplify the abilities of EDTA and CA in modifying the growth regimes of YPO4. Detailed characterizations of the products were implemented by the combined techniques of XRD, FE-SEM, TEM, LPSA (laser particle size analysis), FTIR, and optical spectroscopy, and the mechanisms of the morphology/phase evolution of the YPO4 crystals were investigated via altering the EDTA/Y and CA/Y molar ratios, and the EG content. The particle size-dependent luminescence properties of h-(Y0.95Eu0.05)PO4, including the luminescence intensity, fluorescence lifetime, asymmetry factor of luminescence ((5D0 → 7F2)/(5D0 → 7F1) intensity ratio), and CIE chromaticity coordinates, were also investigated in detail.


 Please wait while we load your content...
Please wait while we load your content...