The role of phosphorylation and dephosphorylation of shell matrix proteins in shell formation: an in vivo and in vitro study†
Abstract
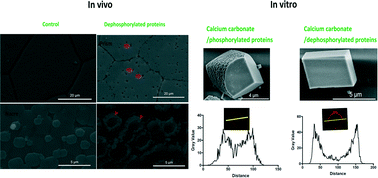
Protein phosphorylation is a fundamental mechanism regulating many aspects of cellular processes. Shell matrix proteins (SMPs) control the crystal nucleation, polymorphism, morphology, and organization of calcium carbonate crystallites during shell formation. SMP phosphorylation is suggested to be important in shell formation but the mechanism is largely unknown. Here, to investigate the mechanism of phosphorylation of SMPs in biomineralization, we performed in vivo and in vitro experiments. By injection of an antibody against the anti-phosphoserine/threonine /tyrosine sites into the extrapallial fluid of the pearl oyster Pinctada fucata, phosphorylation of matrix proteins was significantly reduced after 6 days. Newly formed prismatic layers and nacre tablets were found to grow abnormally with reduced crystallinity and possibly changed crystal orientation shown by Raman spectroscopy. In addition, regeneration of shells is also inhibited in vivo. Then, protein phosphatase was used to dephosphorylate SMPs extracted from the shells. After dephosphorylation, the ability of SMPs to inhibit calcium carbonate formation has been reduced. Surprisingly, the ability of SMPs to modulate the crystal morphology has been largely compromised although the phosphorylation extent remained as at least half that of the control. Furthermore, dephosphorylation of SMPs changed the distribution of protein occlusions and decreased the amount of protein occlusions inside the crystals shown by confocal imaging, indicating the interaction between phosphorylated SMPs and crystals. Taken together, this study provides insight into the mechanism of phosphorylation of SMPs during shell formation.


 Please wait while we load your content...
Please wait while we load your content...