Optimizing crystallinity and porosity of hierarchical Ni(OH)2 through conformal transformation of metal–organic framework template for supercapacitor applications†
Abstract
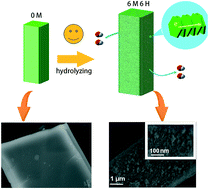
MOFs (metal–organic frameworks) are widely utilized as both the templates and precursors to prepare nanomaterials as supercapacitor electrodes. However, traditional thermolysis routes are difficult to control and usually lead to collapse of the MOF skeletons, which hinders wider applications. Herein, we describe a simple and facile strategy for fabricating the Ni(OH)2 hierarchical structure by the “conformal transformation” of one particular Ni-MOF through hydrolysis in the KOH aqueous solution. By controlling the concentration of KOH solution and the soaking time, the obtained Ni(OH)2 nanomaterial possesses both optimized crystallinity and porosity, extremely beneficial for electron transportation and ion migration within the electrodes. Taking these advantages, the Ni(OH)2 electrode presents a remarkable specific capacity of 830.6 C g−1, and the fabricated all-solid state asymmetric supercapacitor presents a remarkable energy density of 37.8 W h kg−1 at a power density of 252.67 W kg−1, which surpasses most Ni(OH)2 based supercapacitor devices. After 15 000 cycles, the capacitance remains at over 93% of the initial value.


 Please wait while we load your content...
Please wait while we load your content...