Room-temperature synthesis, controllable morphology and optical characteristics of narrow-band red phosphor K2LiGaF6:Mn4+
Abstract
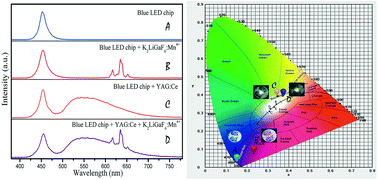
A series of red phosphors based on the substitution of Mn4+ for Ga3+ in the lattice of K2LiGaF6 were synthesized via a co-precipitation method. Their structure, morphology and composition were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy-dispersive X-ray spectrometry (EDS) in detail. The luminescence properties of the K2LiGaF6:Mn4+ samples were systematically evaluated by photoluminescence (PL) spectroscopy. The excitation spectrum consists of two broad bands from 300 to 550 nm. The obtained K2LiGaF6:Mn4+ can exhibit red light peaking at 636 nm for the 2Eg → 4A2g transition of Mn4+ ions under 470 nm excitation. A low concentration quenching phenomenon occurs in K2LiGaF6:Mn4+ and the optimal doping concentration is about 4%. The critical distance was calculated to be 9.107 Å. The changes of the emission intensity and morphology of K2LiGaF6:Mn4+ based on different lithium sources and surfactants were investigated in detail. White light-emitting diodes (WLEDs) fabricated from the red K2LiGaF6:Mn4+ phosphor exhibit good performance with a colour rendering index of 79.6, a low colour temperature of 4378 K and a luminous efficiency of 109.91 lm W−1. Therefore, the K2LiGaF6:Mn4+ red phosphors can be used as good candidates for WLEDs.


 Please wait while we load your content...
Please wait while we load your content...