Intermolecular hydrogen bond stretching vibrations observed in terahertz spectra of crystalline vitamins†
Abstract
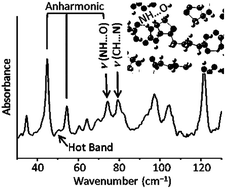
We measured the terahertz spectra of five powdered vitamins, nicotinamide, nicotinic acid, ascorbic acid, pyridoxine hydrochloride, and biotin, to investigate intermolecular hydrogen bonding. These vitamins have various weak to moderate hydrogen bonds between molecules and thus are good candidates for the investigation of various hydrogen bond stretching. Using dispersion-corrected density functional theory calculations on a periodic system, we assigned the observed THz peaks with excellent accuracy. The stretching vibrations of the intermolecular hydrogen bonds are mixed with the intermolecular translational and rotational modes. Depending on the strength of the hydrogen bond and the mass of the moiety linked via the hydrogen bond, the wavenumber of each type of hydrogen bond is located within a limited region. According to the present study, the stretching vibration of a weak hydrogen bond located at approximately 60–105 cm−1 is summarized as having a sharp full width at half maximum of 1–2 cm−1 and the molar extinction coefficient of 4–10 M−1 cm−1. The anharmonicity of the potential energy curve was found in modes containing weak intermolecular NH⋯N and NH⋯O stretching vibrations. Present research on the specific properties of hydrogen bond stretching vibrations observed in the THz region provides useful information for quickly estimating the existence of intermolecular hydrogen bonds.


 Please wait while we load your content...
Please wait while we load your content...