Supramolecular cooperative interaction-induced assembly of phosphotungstate polyanions and sulfur-containing pyridine-based cations†
Abstract
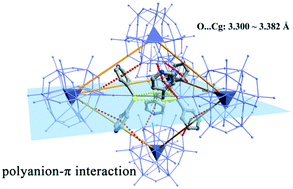
Three new polyoxometalate (POM) supramolecular assemblies constructed from phosphotungstate clusters and S-containing pyridine-based cations had been successfully isolated and characterized. Structural analysis revealed that their formula were (H2PySSPy)3[PW12O40]2·6H2O (1), (H3/2PySPy)2[PW12O40]·6H2O (2) and [Ag(HPyS)]4[Ag8(H2O)9][P2W18O62]2·4H2O (3) (PySSPy = 4,4′-dipyridine disulfide; PySPy = 4,4′-thiodipyridine; PySH = pyridine-4-thiol), respectively. The three crystals were assembled by means of multiple weak intermolecular cooperative interactions of hydrogen bonding, S⋯O, C–H⋯π, etc. In particular, the peculiar supramolecular bond of the ‘polyanion–π’ binding interaction between the polyanion receptor and the six-membered pyridine ring was described in some detail. These complex and noncovalent contacts efficiently induce the construction of the crystal supramolecular lattices of 1–3. During the formation process of 2 and 3, organic moieties were achieved through the in situ cleavage of S–S chemical bonds in PySSPy. Crystal 3 contains a 3D network constructed from Dawson-like polyanions and Ag–PyS cationic units through Ag–O and Ag–S coordination bonds. One anionic [P2W18O62]6− cluster is bonded to eighteen Ag ions through many Ag–O interactions, presenting an interesting Ag18-supported Dawson structure.


 Please wait while we load your content...
Please wait while we load your content...