A novel carbazole derivative containing fluorobenzene unit: aggregation-induced fluorescence emission, polymorphism, mechanochromism and non-reversible thermo-stimulus fluorescence†
Abstract
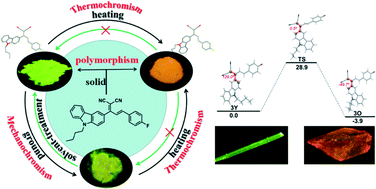
Herein, a novel carbazole derivative ((E)-2-(1-(9-butyl-9H-carbazol-3-yl)-3-(4-fluoro-phenyl)allylidene)malononitrile (3)) containing multi-cyano group and 4-fluoro-benzene unit was synthesized. This compound displayed aggregation-induced emission properties owing to the highly twisted conformation. It exhibited multi solid-state fluorescence emissions (yellow (3Y) and orange (3O)) and two crystalline polymorphs. Through single crystal structural analysis, we observed compound 3 with different conformations and variable molecular packing modes, which resulted in different solid-state fluorescence emissions, polymorphism, mechanochromic behaviors and unique non-reversible thermo-stimulus fluorescence. The fluorescence emission of the yellow solid displayed distinct red-shift after grinding, which is attributed to the breakup of the crystal structure, while that of the orange solid was not evident due to its stable and ordered molecular packing. Additionally, the yellow solid (3Y) also exhibited thermo-stimulus fluorescence, turning into 3O, and crystal phase transition. The lower energy and the compact packing modes of 3O should be collectively responsible for the unique behavior.


 Please wait while we load your content...
Please wait while we load your content...