A salt or a co-crystal – when crystallization protocol matters
Abstract
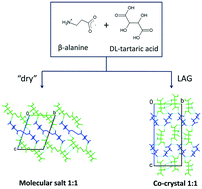
The problem of obtaining multi-component crystals as co-crystals (with neutral molecules) rather than as salts (with charged cations and anions) attracts much attention. This is not merely a scientific challenge, but is often important for issues related to intellectual property in the pharmaceutical industry. Until now, examples have been documented where control over co-crystal–salt state has been achieved by modifying either the chemical components (co-formers) or temperature. Serendipitously we recently obtained, for the first time, a co-crystal and a salt of the same chemical composition – β-alanine and DL-tartaric acid – crystallizing stochastically at the same temperature from the same solution (E. A. Losev and E. V. Boldyreva, Acta Crystallogr., Sect. C: Struct. Chem., 2018, 74, 177–185, 10.1107/S2053229617017909). Here we report the possibility of obtaining reproducibly crystals of either of the two forms, the stable co-crystal (II) or a metastable salt (III), depending on the crystallization protocol. These observations are rationalized in terms of control over nucleation and the nuclei growth of the two phases, Ostwald's rule of stages and “disappearing polymorphs”. We report the results of using slow evaporation, fast and slow anti-solvent crystallization, and co-grinding with a variable amount of added water, as well as of “slurry experiments”. The thermodynamically stable co-crystal (II) can be obtained as a pure phase through liquid-assisted grinding, and upon crystallization from solution if seeds of it are already present in solution. The metastable molecular salt (III) is formed as a pure phase upon “dry” co-grinding without any water added specially (although in a humid atmosphere), upon fast anti-solvent crystallization, or during slow anti-solvent crystallization experiments before any seeds of the co-crystal (II) become available. After the co-crystal (II) has been formed once, even introducing a seed of the molecular salt (III) does not help to crystallize the salt from solution. The salt (III) is thus a typical “disappearing polymorph”. For comparison, we describe the co-crystallization of DL-tartaric acid with other amino acids of the same homological series. This gives the same products under all of the tested crystallization conditions – salts for the larger γ- and α-aminobutyric acids (GABA (IV) and AABA (V), respectively), and a co-crystal for the smaller glycine (I). The findings shed light on the mechanism of the alternative precipitation of co-crystals or salts of zwitterionic compounds from their aqueous solutions.


 Please wait while we load your content...
Please wait while we load your content...