Probing halogen⋯halogen interactions via thermal expansion analysis†
Abstract
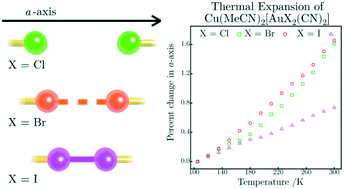
A unique method of probing the existence of X⋯X interactions is described using the isomorphous series Cu(MeCN)2[AuX2(CN)2] (X = Cl, 1; Br, 2; I, 3). These 1-D coordination polymers form a zig-zag chain with X⋯X distances of 4.1913(16), 3.7489(5) and 3.6734(5) Å along the c-axis as nearest neighbor contacts between chains for Cl, Br and I (respectively), corresponding to distances well-beyond (Cl), slightly longer (Br) than and well-below (I) their sums of van der Waals radii. Both 1 and 2 exhibited very high thermal expansion coefficients, reaching ‘colossal’ positive values of 155(2) ppm K−1 and negative values down to −83.3(14) ppm K−1, attributable to the loose packing and flexibility of the 1-D zig-zag chains making up the materials. Compound 3 exhibits significantly reduced thermal expansion coefficients in all directions, reaching a maximum magnitude of 90.8(7) ppm K−1, resulting from the I⋯I interactions holding the chains together, thus restricting expansion. Thus, the presence or absence of X⋯X interactions has a measurable impact on the thermal expansion properties.


 Please wait while we load your content...
Please wait while we load your content...