Recurrent supramolecular motifs in discrete complexes and coordination polymers based on mercury halides: prevalence of chelate ring stacking and substituent effects†
Abstract
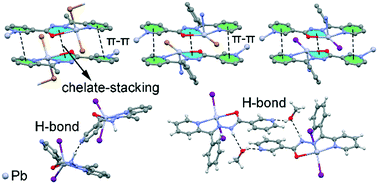
In recent years, the crystal engineering library has been enriched with a number of previously unrecognized or unnoticed intermolecular interactions, such as agostic, tetrel, chalcogen, pnicogen bonding and chelate ring stacking – collectively referred to as “unconventional interactions”. Many open questions remain unaddressed regarding their ability to form synthon interactions, specificity, and cooperativity, for example with π–π stacking interactions. In this work, we throw light on the formation of chelate ring stacking in metal–organic assemblies of nicotinohydrazide ligands (N′-(1-(2-pyridyl)ethylidene)nicotinohydrazide (HL) and N′-(phenyl(pyridin-2-yl)methylene)nicotinohydrazide (HL1)) with mercury(II) halide (HgBr2, HgI2) salts. Their reaction produced five compounds, namely [Hg(μ-L)BrHgBr2]n (1), [Hg(μ-L1)Br]n (2), [Hg(L)I2] (3), [Hg(HL1)I2]·(CH3OH) (4), and [Hg(μ-L1)I]n (5). Crystal structure analysis reveals that chelate ring stackings are formed in four of the reported metal–organic compounds, and are common also in the literature precedents. The energies of chelate ring stackings and π–π heterocycle stackings have been computed and analyzed by means of DFT calculations, and the results were verified using Bader's theory of “atoms in molecules”. These results provide a rationale for preferential formation of both unconventional and conventional stackings and allow us to conclude that chelate ring interaction may be considered as a synthon interaction for nicotinohydrazide metal complexes. Interpretations for packing differences imposed by the substituent effect (substitution of methyl group in HL for phenyl group in HL1) were provided based on the Hirshfeld surface analysis and 2D fingerprint plots of the crystal structures reported here.


 Please wait while we load your content...
Please wait while we load your content...