Weak interactions cause selective cocrystal formation of lanthanide nitrates and tetra-2-pyridinylpyrazine†
Abstract
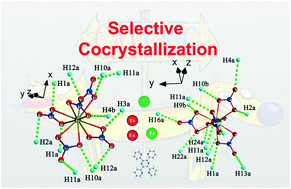
Two series of cocrystals with the formula Ln(NO3)5·H2TPPZ (Ln = Eu, Tb, Er and Y, TPPZ = 2,3,5,6-tetra-2-pyridinylpyrazine) have been designed and synthesized. Utilizing single crystal structure parsing, we demonstrate how weak hydrogen bonding interactions between lanthanide nitrates and organic molecules stabilize their cocrystal structures. The lanthanide contraction effect is also proposed to help determine the Ln–O bond regularity, hence rationalizing the stability and structural variation of the cocrystals. Furthermore, the luminescence properties of these cocrystals were analyzed in detail. We found an interesting result of decent red fluorescence of the 1-Eu cocrystal complex.


 Please wait while we load your content...
Please wait while we load your content...