Water mediated proton conductance in a hydrogen-bonded Ni(ii)-bipyridine-glycoluril chloride self-assembled framework†
Abstract
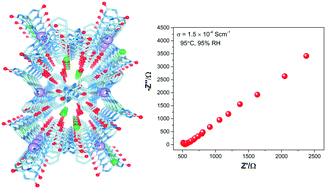
Proton conducting properties have been investigated in a new Ni(II)-based hydrogen-bonded porous framework synthesized using a urea-fused bipyridine-glycoluril (BPG) tecton. This hydrogen-bonded self-assembled structure encapsulates water molecules in the channels with hydrogen-bonding networks which exhibits a significant temperature dependent proton conductance of 1.5 × 10−4 S cm−1 at 95 °C and 95% RH with a low activation energy (Ea) of 0.54 eV, implying a Grotthuss proton hopping mechanism mediated by hydrogen-bonded water molecules in the channels. In addition, this framework exhibited a very high water uptake under humid conditions. A continuous array of water molecules and chloride ions embedded in the highly hydrophilic porous channels of the hydrogen-bonded framework acts as the proton conducting medium.


 Please wait while we load your content...
Please wait while we load your content...