Catalyst-free and visible light promoted trifluoromethylation and perfluoroalkylation of uracils and cytosines†
Abstract
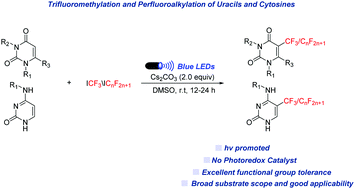
Fluoroalkylated enaminones, such as trifluridine and 5-trifluoromethyluracil, have widespread applications in pharmaceuticals and agrochemicals. Although these kinds of pharmaceutical agent often bear CF3 and perfluoroalkyl motifs in the core structure, access to such analogues typically requires multi-step synthesis. Here, we report a mild, metal-free and operationally simple strategy for the direct perfluoroalkylation of uracils, cytosines and pyridinones through a visible-light induced pathway from perfluoroalkyl iodides. This photochemical transformation features synthetic simplicity, mild reaction conditions without any photoredox catalyst, and high functional group tolerance, providing a facile route for applications in medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...