Highly enantioselective addition of dimethylzinc to fluorinated alkyl ketones, and the mechanism behind it†
Abstract
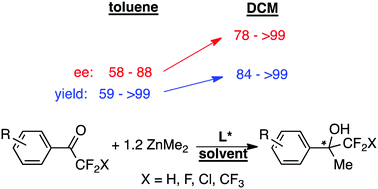
Chiral-diamine catalyzed addition of ZnMe2 to PhC(O)CF2X (in dichloromethane at −30 °C) affords fluorinated alkyl tertiary alcohols in high yield (quantitative for X = H, F, Cl; 84% for X = CF3) and up to 99% ee. These conditions are similarly very efficient for other various ArC(O)CF3 molecules. A fine analysis of the results can be performed based on a double-cycle mechanism.


 Please wait while we load your content...
Please wait while we load your content...