Diastereoselective synthesis of 1,3-disubstituted isoindolines and sultams via bronsted acid catalysis†
Abstract
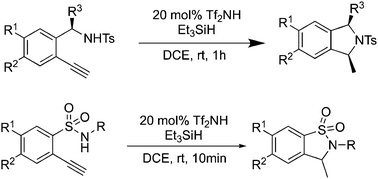
The bis(trifluoromethane)sulfonimide (Tf2NH) catalyzed intramolecular hydroamidation of terminal alkynes is reported. The combination of Et3SiH and Tf2NH provides cis-1,3-disubstituted isoindolines and sultams in high yield (up to 98%) and high diastereoselectivity (up to 99 : 1 d.r.).


 Please wait while we load your content...
Please wait while we load your content...
