Csp3–H bond functionalization of amines via tunable iminium ions: divergent synthesis of trifluoromethylated arylamines†
Abstract
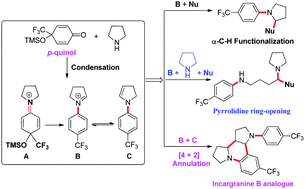
A series of tunable iminium ions, generated in situ by the condensation of 4-trifluoromethyl-p-quinols with cyclic amines, can lead to the divergent synthesis of trifluoromethylated arylamines in a single step via redox-neutral isomerization. The direct α- and β-functionalization of saturated amines can be achieved regioselectively under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...