Immunoglobulin light chain amyloid aggregation
Abstract
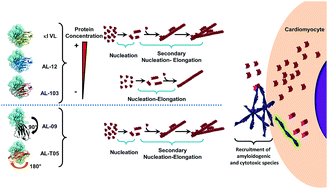
Light chain (AL) amyloidosis is a devastating, complex, and incurable protein misfolding disease. It is characterized by an abnormal proliferation of plasma cells (fully differentiated B cells) producing an excess of monoclonal immunoglobulin light chains that are secreted into circulation, where the light chains misfold, aggregate as amyloid fibrils in target organs, and cause organ dysfunction, organ failure, and death. In this article, we will review the factors that contribute to AL amyloidosis complexity, the findings by our laboratory from the last 16 years and the work from other laboratories on understanding the structural, kinetics, and thermodynamic contributions that drive immunoglobulin light chain-associated amyloidosis. We will discuss the role of cofactors and the mechanism of cellular damage. Last, we will review our recent findings on the high resolution structure of AL amyloid fibrils. AL amyloidosis is the best example of protein sequence diversity in misfolding diseases, as each patient has a unique combination of germline donor sequences and multiple amino acid mutations in the protein that forms the amyloid fibril.
- This article is part of the themed collection: Amyloid Aggregation


 Please wait while we load your content...
Please wait while we load your content...