Charge effects regulate reversible CO2 reduction catalysis†
Abstract
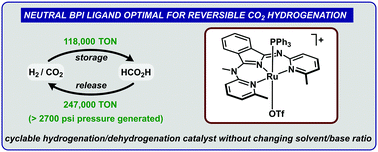
Modular but geometrically constrained ligands were used to investigate the impact of key ligand design parameters (charge and bite angle) on CO2 hydrogenation and formic acid dehydrogenation activity. These studies yielded an optimized catalyst that achieved over 118 000 turnovers in CO2 hydrogenation, 247 000 turnovers in HCO2H dehydrogenation, was applied in a hydrogen storage device used for 6 cycles of hydrogen storage/release without requiring changes in pH or solvent, and generated H2/CO2 gas at a pressure of 190 atm from formic acid.


 Please wait while we load your content...
Please wait while we load your content...
