Revisiting copper reduction in zeolites: the impact of autoreduction and sample synthesis procedure†
Abstract
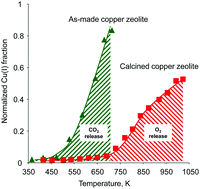
At least two distinct processes occur during heating of the copper-exchanged mordenite in oxygen-free environment depending on the sample synthesis procedure. The first process corresponds to the reaction of Cu(II) sites with the residual traces of carbonaceous impurities left in the sample after the synthesis without calcination in an oxidant-containing environment, yielding Cu(I) and carbon dioxide. The second relates to autoreduction of Cu(II) into Cu(I) species accompanied with the release of molecular oxygen. The results show that to completely remove all traces of carbonaceous impurities and to avoid the loss of the active Cu(II) sites during the activation, copper-exchanged zeolite samples should be calcined in oxidative gases at elevated temperature and, preferentially, prior to the experiments to avoid the adsorption of organic compounds from air.


 Please wait while we load your content...
Please wait while we load your content...