Dehydroxylation of alcohols for nucleophilic substitution†
Abstract
The Ph3P/ICH2CH2I system-promoted dehydroxylative substitution of alcohols was achieved to construct C–O, C–N, C–S and C–X (X = Cl, Br, and I) bonds. Compared with the previous approaches such as the Appel reaction and Mitsunobu reaction, this protocol offers some practical advantages such as safe operation and a convenient amination process.
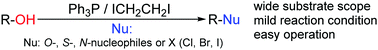


 Please wait while we load your content...
Please wait while we load your content...