An aryne triggered ring-opening fluorination of cyclic thioethers with potassium fluoride†
Abstract
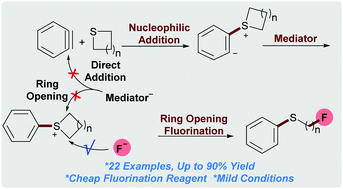
Herein, we report an aryne triggered ring-opening fluorination protocol of a great variety of saturated sulfur heterocycles. A key factor for the success is the identification of a suitable mediator. Compared to previous methods, this transition-metal free protocol employs low-cost potassium fluoride as the fluorine source. The operational simplicity and mild reaction conditions allow for the rapid synthesis of a wide range of aliphatic fluoride compounds in good yields.


 Please wait while we load your content...
Please wait while we load your content...