Direct synthesis of N-terminal thiazolidine-containing peptide thioesters from peptide hydrazides†
Abstract
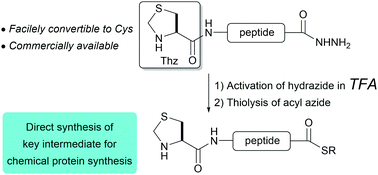
We report a simple and promising synthetic method to oxidize peptide hydrazides containing N-terminal thiazolidine as a protected cysteine. This yields the corresponding thioester via a peptide azide without decomposition of the thiazolidine ring. The newly developed protocol was validated by the synthesis of the bioactive peptide LacZα.


 Please wait while we load your content...
Please wait while we load your content...