Synthesis of functionalized pyrroloindolines via a visible-light-induced radical cascade reaction: rapid synthesis of (±)-flustraminol B†
Abstract
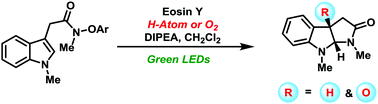
The development of visible-light-induced synthesis of functionalized pyrroloindolines via a radical cascade reaction is reported. The reaction shows a broad substrate scope and highlights the mild nature of the reaction conditions. A range of substitutions on indole aromatic rings and N-centers is well tolerated, including a free allylic alcohol. Relying on the strategy, a rapid synthesis of (±)-flustraminol B was achieved.


 Please wait while we load your content...
Please wait while we load your content...