C–H bond sulfonylation of anilines with the insertion of sulfur dioxide under metal-free conditions†
Abstract
C–H bond sulfonylation of anilines with the insertion of sulfur dioxide under metal-free conditions is described. 2-Sulfonylanilines are generated in moderate to good yields through a three-component reaction of anilines, DABCO·(SO2)2, and aryldiazonium tetrafluoroborates under mild conditions. No metal catalysts or additives are needed for this transformation. This direct C–H functionalization is highly efficient, and broad functional group tolerance is observed. A radical process is believed to be involved. In the reaction process, the arylsulfonyl radical and the tertiary amine radical cation generated in situ from DABCO·(SO2)2, and aryldiazonium tetrafluoroborate are the key intermediates. Additionally, the tertiary amine radical cation acts as the electron carrier through a single electron transfer process.
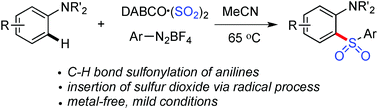


 Please wait while we load your content...
Please wait while we load your content...