Pd(ii)-Catalyzed gamma-C(sp3)–H alkynylation of amides: selective functionalization of R chains of amides R1C(O)NHR†
Abstract
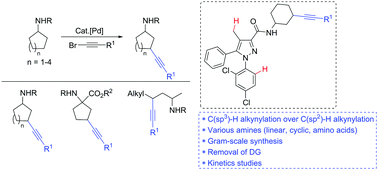
The gamma C(sp3)–H bond alkynylation of R chains of amides R1C(O)NHR, a fundamental class of synthetic substrates, has not been accomplished to date. Here, the first example of palladium(II)-catalyzed alkynylation of an unactivated gamma C(sp3)–H bond of alkyl amides (cyclic, linear, and amino acids) is reported. The kinetic experiment shows that the rate of the reaction depends on the coupling partners and the amides. Late-stage diversification of alkynylated amides was developed by utilizing amine and alkyne functionalities.


 Please wait while we load your content...
Please wait while we load your content...