Selective formation of heterocyclic trans-cycloalkenes by alkyne addition to a biphenylene-based phosphane/borane frustrated Lewis pair†
Abstract
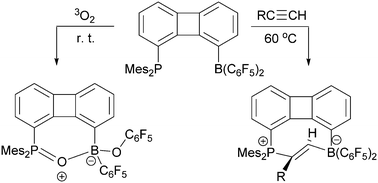
The intramolecular 1-PMes2/8-B(C6F5)2 substituted biphenylene frustrated Lewis pair 4 shows some behavior reminiscent of intermolecular FLP systems. It undergoes trans-1,2-addition to a series of 1-alkynes to give the respective heterocyclic eight-membered E-alkenes 8. The P/B FLP 4 also reacts with triplet dioxygen to yield the [P]–O–[B](OC6F5) containing oxygenation product.


 Please wait while we load your content...
Please wait while we load your content...