Transition metal-free one-pot double C–H functionalization of quinolines with disubstituted electron-deficient acetylenes†
Abstract
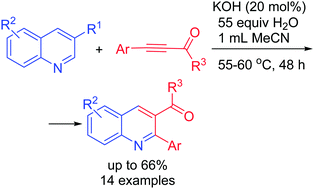
Transition metal-free one-pot reaction of quinolines with acylarylacetylenes and water proceeds in the presence of KOH (55–60 °C, MeCN, 48 h) to afford 2-aryl-3-acylquinolines in up to 66% yield. Here, a formal replacement of the acetylene moiety by the aryl and acyl substituents in the quinoline scaffold takes place. In fact, it has been proved experimentally that the reaction involves the ring cleavage, accompanied by the rearrangement and insertion of the electron-deficient acetylene moiety to form a dihydroquinoline intermediate with an aldehyde functional group in position 4. This intermediate gives the corresponding doubly functionalized quinolines.


 Please wait while we load your content...
Please wait while we load your content...