Structural mechanisms of oligomer and amyloid fibril formation by the prion protein
Abstract
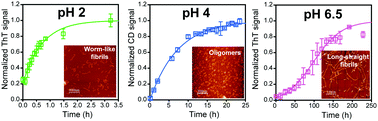
Misfolding and aggregation of the prion protein is responsible for multiple neurodegenerative diseases. Works from several laboratories on folding of both the WT and multiple pathogenic mutant variants of the prion protein have identified several structurally dissimilar intermediates, which might be potential precursors to misfolding and aggregation. The misfolded aggregates themselves are morphologically distinct, critically dependent on the solution conditions under which they are prepared, but always β-sheet rich. Despite the lack of an atomic resolution structure of the infectious pathogenic agent in prion diseases, several low resolution models have identified the β-sheet rich core of the aggregates formed in vitro, to lie in the α2–α3 subdomain of the prion protein, albeit with local stabilities that vary with the type of aggregate. This feature article describes recent advances in the investigation of in vitro prion protein aggregation using multiple spectroscopic probes, with particular focus on (1) identifying aggregation-prone conformations of the monomeric protein, (2) conditions which trigger misfolding and oligomerization, (3) the mechanism of misfolding and aggregation, and (4) the structure of the misfolded intermediates and final aggregates.
- This article is part of the themed collection: Amyloid Aggregation


 Please wait while we load your content...
Please wait while we load your content...