Carbene-catalyzed enal γ-carbon addition to α-ketophosphonates for enantioselective access to bioactive 2-pyranylphosphonates†
Abstract
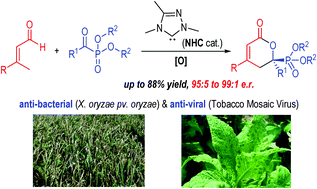
A carbene-catalyzed enantioselective [4+2] cycloaddition reaction between α,β-unsaturated aldehydes and α-ketophosphonates is developed. The reaction affords chiral 2-pyranylphosphonates with excellent enantioselectivities. The optically enriched phosphonate products bear multiple functional groups, including unsaturated lactone and phosphonate moieties that often lead to unique bio-activities. Preliminary studies show that the products from our reactions exhibit anti-bacterial (X. oryzae pv. oryzae) and anti-viral (Tobacco Mosaic Virus) activities for potential use in plant protection.


 Please wait while we load your content...
Please wait while we load your content...