Temperature controlled formation of polar copper phosphonates showing large dielectric anisotropy and a dehydration-induced switch from ferromagnetic to antiferromagnetic interactions†
Abstract
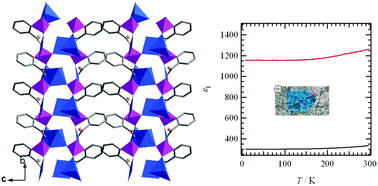
The reaction of copper(II) chloride with 2-carboxyphenylphosphonic acid (2-cppH3) under hydrothermal conditions at 100 °C results in the formation of a polar compound, Cu3(2-cpp)2(H2O)5 (1), which shows large dielectric anisotropy. A similar reaction at 140 °C results in a centrosymmetric phase, Cu3(2-cpp)2(H2O)2 (2). Compound 1 can convert into 2 upon increasing the reaction temperature under hydrothermal conditions, and further to Cu3(2-cpp)2 (3) upon thermal treatment at 240 °C. This process is accompanied by a switch from ferromagnetic in 1 to antiferromagnetic interactions in 2 and 3.


 Please wait while we load your content...
Please wait while we load your content...