Orthogonal, dual protein labelling by tandem cycloaddition of strained alkenes and alkynes to ortho-quinones and azides†
Abstract
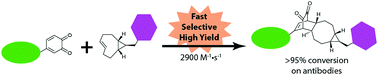
Reaction of cyclopropanated trans-cyclooctene (cpTCO) with in situ generated ortho-quinone is an efficient tool for bioorthogonal protein conjugation. The (4+2)-cycloaddition of cpTCO with ortho-quinone is significantly faster than its cyclooctyne counterpart (BCN). Orthogonal, tandem cpTCO–quinone and BCN–azide cycloadditions afforded a homogeneous, dual labelled antibody–drug conjugate.


 Please wait while we load your content...
Please wait while we load your content...