Electron transfer-induced reduction of organic halides with amines†
Abstract
Reduction of a variety of organo halides was examined by using amines as a sacrificial hydrogen source. UV light-induced reduction of vinyl and aryl halides with triethylamine proceeded smoothly to give the corresponding reduced products. High temperature heating also caused the reduction and DABCO (1,4-diazabicyclo[2.2.2]octane) also served as a good reducing reagent.
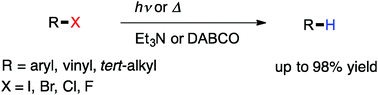


 Please wait while we load your content...
Please wait while we load your content...